Pathogenesis of Type 1 Diabetes and Imaging
Type 1 diabetes is a heterogeneous inflammatory disease characterized by the infiltration of pancreatic islets by autoimmune cells.
The progression of autoimmune cell infiltration in the pancreas promotes insulin-producing β-cell dysfunction (reduced first-phase insulin response) and later β-cell elimination, ultimately resulting in the onset of diabetes. Unfortunately, there are no clinically approved diagnostic imaging techniques to monitor disease progression and interventional therapies for treating underlying autoimmunity and boosting β-cell survival in type 1 diabetes.
Group Leader, Esteban Gurzov, Ph.D.
Research Interests
The role of protein tyrosine phosphatases in β-cell dysfunction and the development of type 1 diabetes.
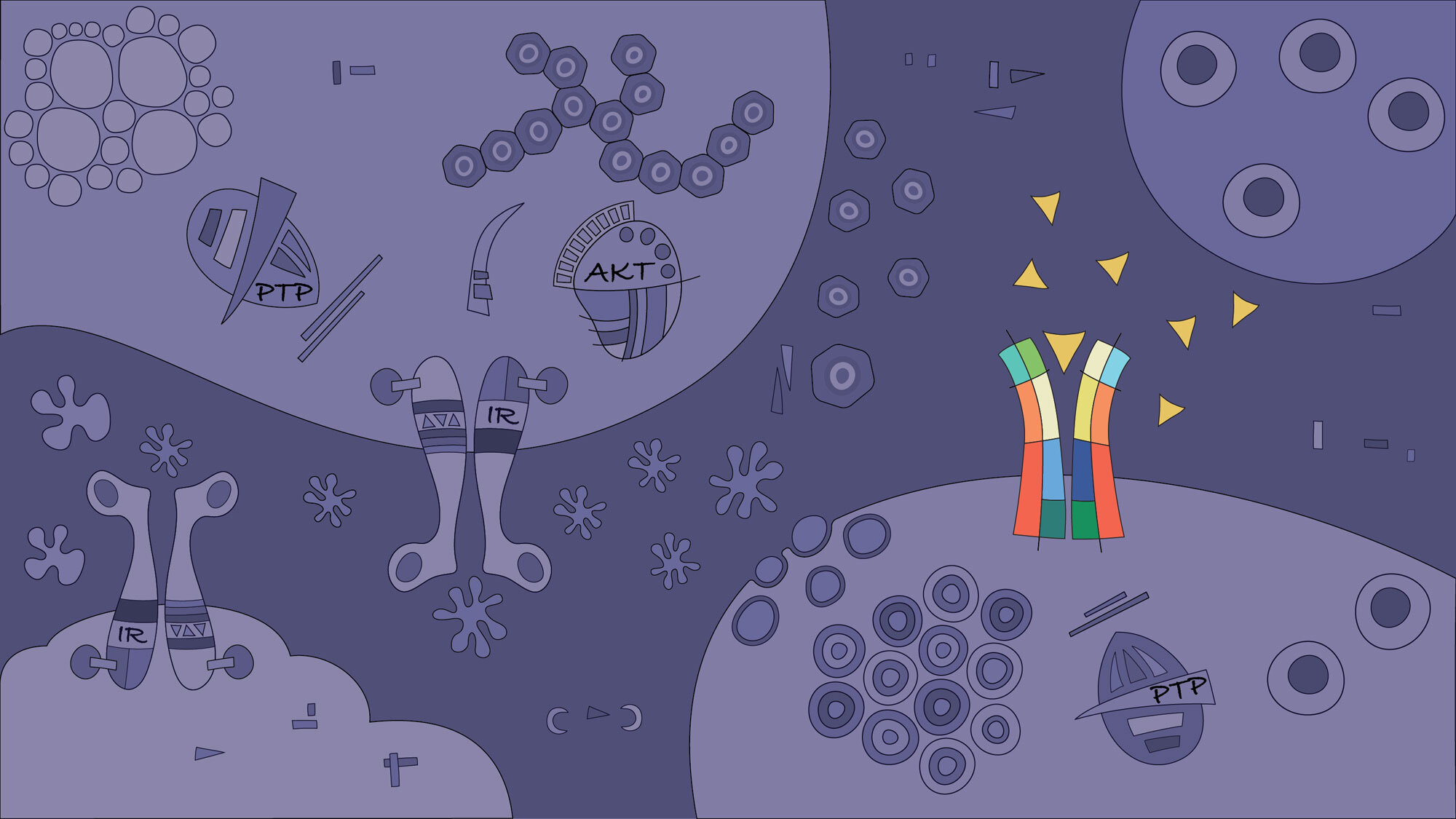
In this project, we aim to characterise the impact of pancreatic β-cell inactivation of protein tyrosine phosphatases (PTPs). Single-nucleotide polymorphisms in members of this group of phosphatases, such as PTPN2 and PTPN22, are associated with autoimmune diseases, including type 1 diabetes. Our studies demonstrated that PTPs are also expressed in pancreatic islets and β-cells. We have shown that the inactivation of phosphatases by inflammatory-mediated oxidative stress severely affects pancreatic β-cell function and survival in patients with type 1 diabetes. Testing our hypothesis that PTP dysregulated activity is a common mechanism in autoimmunity will increase our understanding of the pathogenesis and postulate novel targets for future clinical interventions in type 1 diabetes. For instance, identification of the mechanisms by which PTPs modulate cytokine activity may provide relevant and realistic targets for the design of pharmacological agents and diets. This project can explain many “unknowns” that still linger regarding the disease. (Figure adapted from Gurzov EN, et al. TEM 2015)
The quest to “light up” β-cells in the pancreas for an early diagnosis of type 1 diabetes.
Diagnosis and treatment of early type 1 diabetes is currently hampered by a lack of reliable techniques to quantify β-cell mass in the pancreas. β-cell mass is indirectly estimated in the clinic by measuring serum C-peptide (produced at an equimolar ratio with insulin). However, β-cell function and therefore insulin and C-peptide production are impaired in the early stages of type 1 diabetes. Hence, this indirect method does not provide a reliable picture of β-cell mass. As part of our intensive research program to design a β-cell specific probe that can be used to measure β-cell mass, we recently tested novel approaches for the detection of β cells in vivo, including labeled insulin-binding peptides. In this project, we use 3D imaging, bioinformatics analysis, structural biology, in vitro and in vivo techniques to characterise and study the function of additional novel molecules and nanoparticles for β-cell detection. These molecules will hopefully enable for the first time a reliable assessment of β-cell mass in the clinics. (Figure adapted from Gurzov EN, et al. TEM 2020)
Selected Publications
Negueruela J, Vandenbempt V, Talamantes S, Ribeiro-Costa F, Nunes M, Dias A, Bansal M, Gurzov EN. Protocol for CRISPR-Cas12a genome editing of protein tyrosine phosphatases in human pluripotent stem cells and functional β-like cell generation. STAR Protocols 5(3):103297, 2024
Vandenbempt V, Eski SE, Brahma MK, Negueruela J, Bruggeman Y, Demine S, Xiao P, Cardozo AK, Baeyens N, Martelotto LG, Singh SP, Mariño E, Gysemans C, Gurzov EN. HAMSAB diet ameliorates dysfunctional signalling in pancreatic islets in autoimmune diabetes. iScience 27(1):108694, 2023
Elvira B, Vandenbempt V, Bauzá-Martinez J, Crutzen R, Negueruela J, Ibrahim H, Winder ML, Brahma MK, Vekeriotaite B, Martens PJ, Singh SP, Rossello F, Lybaert P, Otonkoski T, Gysemans C, Wu W, Gurzov EN. PTPN2 Regulates the Interferon Signaling and Endoplasmic Reticulum Stress Response in Pancreatic β-Cells in Autoimmune Diabetes. Diabetes 71(4):653-668, 2022
Eriksson M, Litwak SA, Yun Y, Stanley WJ, Thorn P, Ahlgren U, Gurzov EN. Insulin-Binding Peptide Probes Provide a Novel Strategy for Pancreatic β-Cell Imaging. Mol Pharm online, 2021
Gurzov EN, Ke PC, Ahlgren U, Garcia Ribeiro RS, Gotthardt M. Novel Strategies to Protect and Visualize Pancreatic β Cells in Diabetes. Trends Endocrinol Metab 31(12):905-917, 2020
Stanley WJ, Trivedi PM, Sutherland AP, Thomas HE, Gurzov EN. Differential regulation of pro-inflammatory cytokine signalling by protein tyrosine phosphatases in pancreatic β-cells. J Mol Endocrinology 59:325-337, 2017
Gurzov EN, Stanley WJ, Brodnicki TC, Thomas HE. Protein tyrosine phosphatases: molecular switches in metabolism and diabetes. Trends Endocrinol Metab 26:30-39, 2015
Stanley WJ, Litwak SA, Quah HS, Tan SM, Kay TW, Tiganis T, de Haan JB, Thomas HE, Gurzov EN. Inactivation of protein tyrosine phosphatases enhances interferon signaling in pancreatic islets. Diabetes 64:2489-2496, 2015
Support our research
You have the opportunity to become a supporter of the work being undertaken at the Signal Transduction & Metabolism Laboratory - your support will help the new generation of junior researchers become experienced scientists and may make a difference in the search of better treatments for diabetes and liver cancer.



